1.4K
The difference between atom and molecule is quickly explained. Both units are important for understanding our world from a chemical point of view.
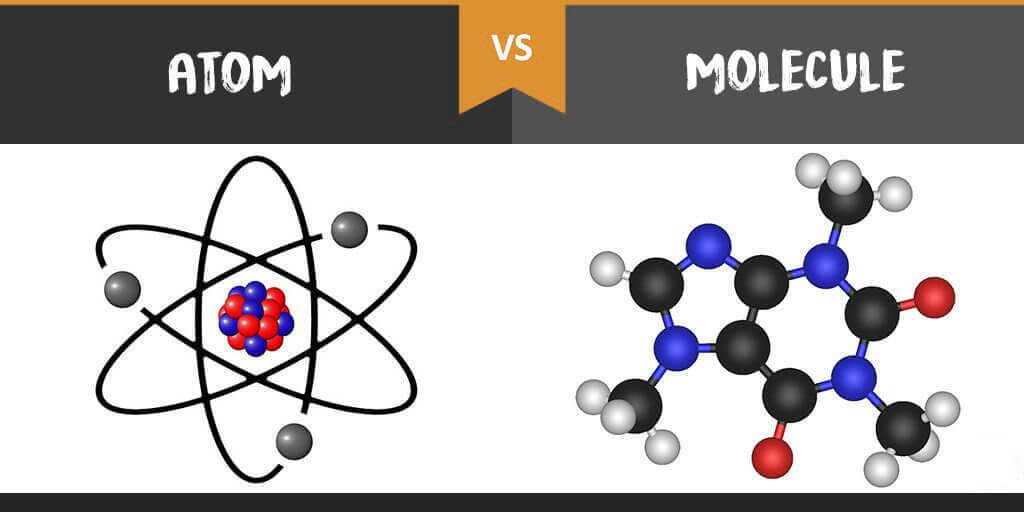
Difference between molecule and atom explained simply
Unlike molecules, atoms are much smaller – because molecules are made of atoms. Molecules are solid atomic bonds. Atoms are the smallest unit into which matter can be divided chemically or mechanically.
- However, atoms are also made up of different particles, protons, neutrons and electrons.
- Every atom has a nucleus. This is where the positively charged protons and the neutral neutrons are located.
- The nucleus is orbited by the negative electrons. They move on different planes called shells.
This is how the fixed connection is created
Atoms have different numbers of shells depending on their number of electrons. A maximum of eight electrons fit on each shell. The atoms – you can imagine – are distributed from the inside to the outside of the shells.
- The outermost, the so-called valence shell, is accordingly usually not fully occupied. However, in order to have eight electrons in their valence shell, atoms form compounds. We then call these molecules.
- Stable compounds are formed when atoms are combined so that each atom in the compound has exactly eight valence electrons, but some of these are shared with other atoms.
- A well-known example: An oxygen atom is missing two electrons on the valence shell. Therefore, it forms a compound with two hydrogen atoms, each of which brings along one electron. The compound is called H2O, better known as water.