1.2K
A battery stores chemical energy and releases electrical energy. But how exactly does it actually work? We’ll explain it to you here.
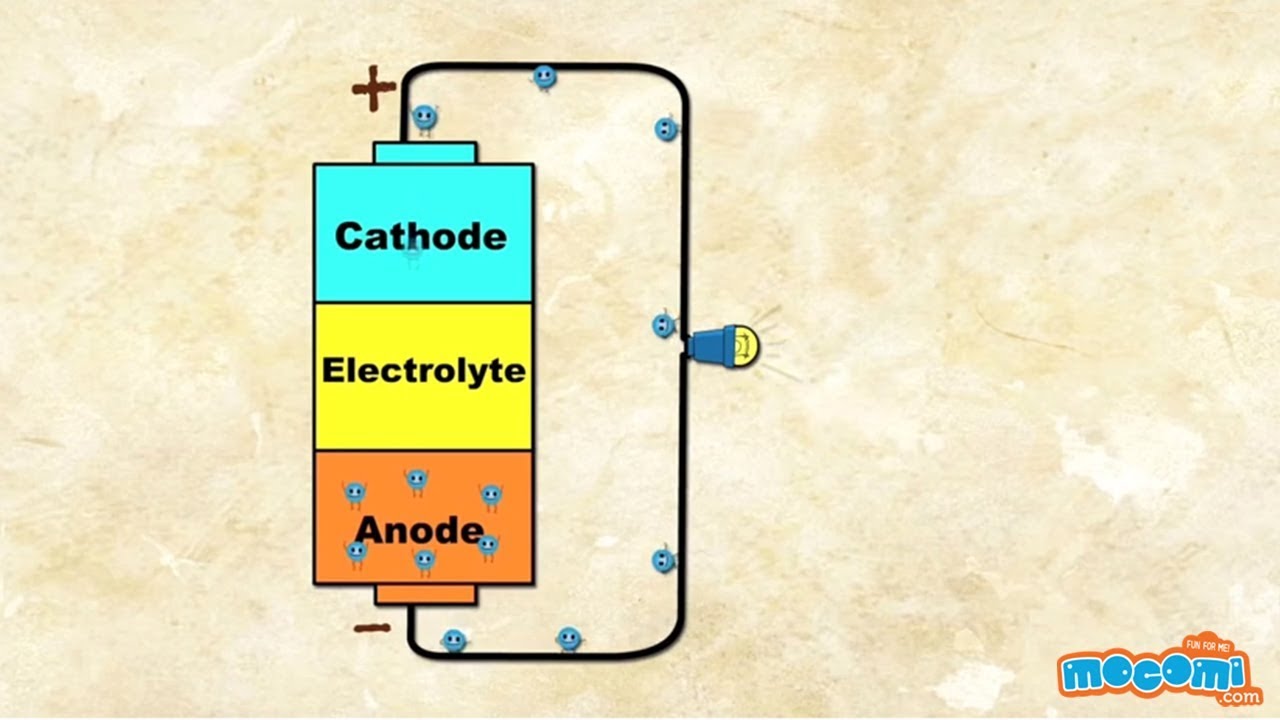
Construction of a battery
For a better understanding of how a battery works, here is a brief explanation of its construction. The most common type is the so-called alkaline manganese battery, the structure and function of which we explain to you in this article as an example.
- A battery consists of one or more galvanic cells, i.e. cells that can store chemical energy and release electrical energy.
- The battery is enclosed in a metal casing that acts as a positive terminal. The bottom of the casing is open for the time being.
- At the edge of the battery is manganese, which is the positive electrode, also called the cathode.
- Separated from the cathode by a separator, for example made of a paper-like material, is the negative electrode, the anode. It is made of zinc.
- Both areas, cathode and anode, are impregnated with caustic potash solution. It forms an electrolyte that improves the conductivity of the materials.
- In the zinc mass in the middle of the battery is a metal pin that makes contact with a metal plate on the bottom.
- The metal plate on the underside forms the negative terminal and seals the battery off at the bottom. It is separated from the positive terminal and the cathode by an insulator.
Function of a battery
When a battery is inserted into a device, a circuit is created. For example, when a light bulb is connected, electrons flow from the negative terminal through the filament to the positive terminal. But why does this happen?
- Zinc is a metal that chemists call base metal. It tends to give off electrons.
- Manganese, on the other hand, is a noble metal, it accepts electrons.
- When the two areas, cathode and anode, are connected to each other, the zinc, which is soaked in potassium hydroxide solution, gives up electrons to the manganese via the conductor.
- The electrons in the zinc mass are produced by a reaction of the zinc with the caustic potash solution to form zinc hydroxide and two electrons.
- The zinc hydroxide then oxidises further to zinc oxide and water.
- The battery is empty when all the zinc has oxidised with the caustic potash to zinc oxide and water and has given up all the free electrons.